Brixadi is a long-acting buprenorphine injection for opioid use disorder. It helps manage opioid dependence by reducing cravings and withdrawal symptoms. This guide explains Brixadi’s formulations, how it works, and what patients can expect from treatment.
Key Takeaways
- Brixadi is an FDA-approved extended-release buprenorphine injection, offered in weekly and monthly formulations, aimed at treating moderate to severe opioid use disorder by stabilizing medication levels and reducing cravings.
- The administration of Brixadi requires healthcare supervision through subcutaneous injections, with a necessary initial test dose of transmucosal buprenorphine to assess tolerance before transitioning to extended-release treatment.
- Patient satisfaction and adherence rates with Brixadi are notably high, due to its consistent medication release, flexibility in dosing, and effectiveness in managing withdrawal symptoms and cravings.
What is Brixadi?
Brixadi is an extended-release buprenorphine injection used to treat moderate to severe opioid use disorder (OUD). Key points about Brixadi include:
- It is approved by the FDA.
- Produced by Braeburn Pharmaceuticals.
- Available in two formulations: weekly and monthly.
- Helps individuals manage opioid dependence by maintaining stable medication levels in the body.
- Reduces cravings and withdrawal symptoms.
The flexibility of having both weekly and monthly formulations allows healthcare providers to tailor treatment plans to the needs of each patient, making it a versatile option in the fight against opioid addiction.
How does Brixadi work?
Brixadi works by utilizing buprenorphine, a partial opioid agonist, which binds to the opioid receptors in the brain. Unlike full agonists, buprenorphine partially activates these receptors, which helps to reduce cravings and withdrawal symptoms without producing the same high associated with other opioids. This unique action makes buprenorphine an effective treatment for opioid dependence, as it diminishes the effects of other opioids, helping patients to stay on their recovery path.
The extended-release formulation of Brixadi is designed to function as a long-acting injectable extended-release injection:
- Once administered subcutaneously, the medication converts into a gel-like substance.
- This gel gradually releases buprenorphine over a period of a week or a month, depending on the formulation.
- The slow and steady release mechanism maintains consistent buprenorphine levels in the bloodstream.
- This avoids the peaks and troughs associated with daily dosing.
Clinical studies have demonstrated the effectiveness of Brixadi, showing that it is comparable to daily oral buprenorphine-naloxone in reducing illicit opioid use, especially in the context of long-term use of buprenorphine. Maintaining a stable therapeutic level of buprenorphine, Brixadi effectively manages patient symptoms and supports long-term recovery.
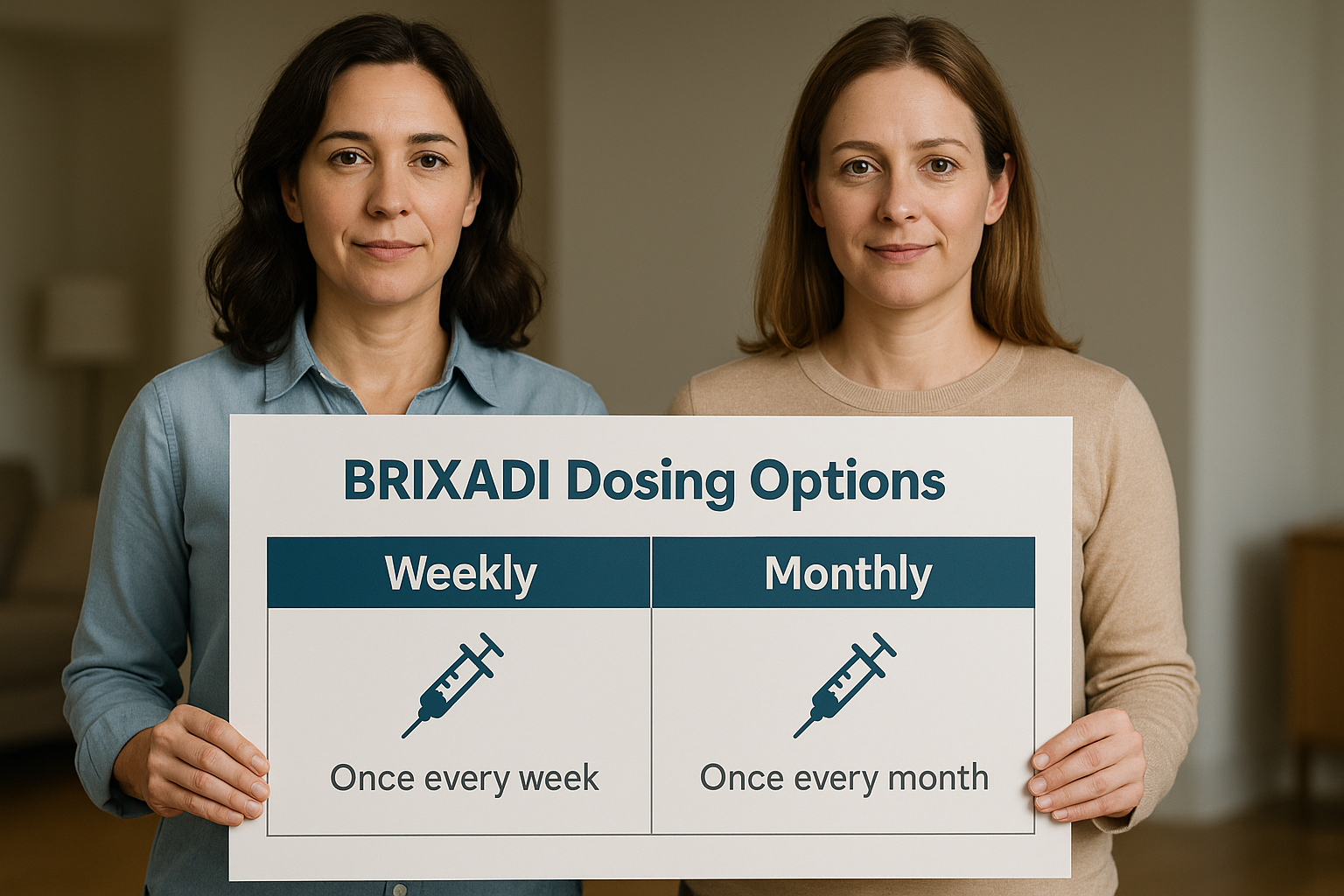
Brixadi dosing options: Weekly vs. Monthly
Brixadi offers two distinct dosing options to cater to different patient needs: weekly and monthly injections. The weekly formulation involves doses administered every seven days, while the monthly dose formulation requires injections every 28 days. This flexibility allows healthcare providers to tailor treatment plans according to patient preferences and clinical requirements, ensuring a more personalized approach to OUD treatment.
Initial dosing for Brixadi typically involves a test dose of transmucosal buprenorphine to ensure the patient’s tolerance before transitioning to the extended-release formulation. For maintenance, patients can either continue on the weekly doses or switch to the monthly doses based on their stability and buprenorphine treatment goals while currently receiving buprenorphine treatment. The ability to seamlessly transition between weekly and monthly doses offers significant advantages for both patients and clinicians, allowing adjustments as recovery progresses.
The choice between weekly and monthly dosing depends on various factors, including the patient’s clinical stability, lifestyle, and convenience. Weekly doses might be preferable for those who require closer monitoring, while monthly doses can offer greater convenience for stable patients. This adaptability ensures that doses of brixadi weekly can accommodate a wide range of patient needs and preferences.
Who is Brixadi for?
Brixadi buprenorphine is designed for adults experiencing moderate to severe opioid use disorder. It is intended for those who have started treatment with a single dose of a transmucosal buprenorphine product or those currently undergoing therapy for opioid addiction. This medication should be part of a comprehensive treatment strategy that includes counseling and support, addressing both the physical and psychological aspects of addiction.
Patients who benefit most from Brixadi include:
- Those who have demonstrated clinical stability and are committed to their treatment goals.
- Individuals who might need more frequent monitoring, for whom brixadi weekly doses might be suitable.
- Those with stable conditions who may prefer the convenience of monthly doses and a dose of brixadi.
However, it is crucial to monitor patients for signs of substance abuse, addiction, and potential misuse due to the opioid analgesic content.
Caution is necessary for certain populations, including individuals with liver disease or those who are pregnant. Brixadi is not suitable for opioid-naïve individuals, as it can pose significant risks. Therefore, a thorough assessment by a healthcare provider is essential to determine if Brixadi is the right treatment option.
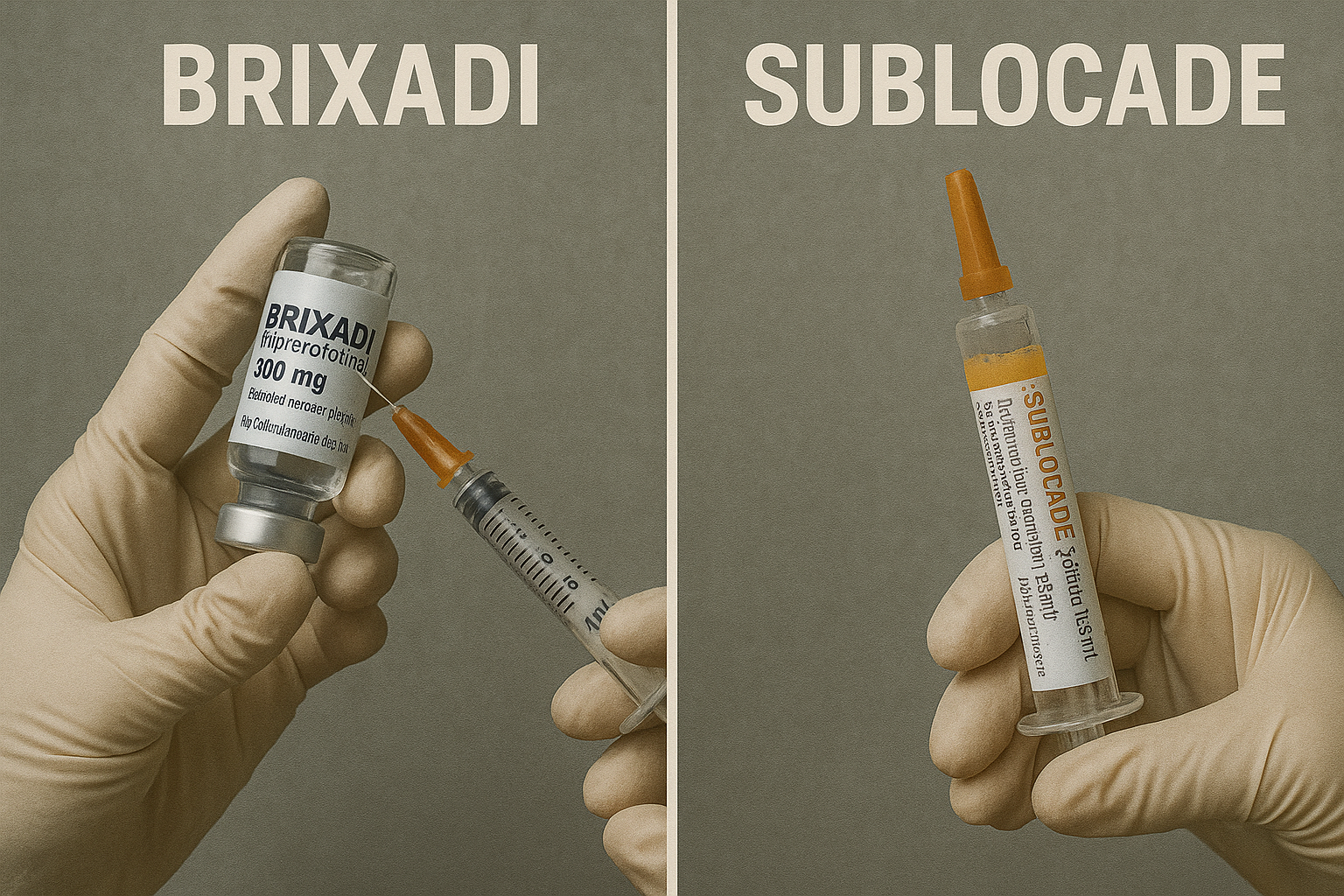
Brixadi vs. Sublocade: Key differences and similarities
When comparing Brixadi vs suboxone, several key differences and similarities emerge, particularly in terms of dosing frequency, administration protocols, and patient suitability. Both medications are administered as subcutaneous injections and use buprenorphine to treat opioid use disorder. However, while Brixadi offers both weekly and monthly dosing options, Sublocade is available only as a monthly injection. This gives Brixadi an edge in terms of dosing flexibility.
In terms of initiation, Brixadi requires a test dose of transmucosal buprenorphine before starting the extended-release formulation, whereas Sublocade does not. The injection sites also differ slightly, with Brixadi administered in the buttock, thigh, abdomen, or upper arm, while Sublocade is typically injected in the abdominal area. Only injectable buprenorphine is an option that differs from these formulations.
Insurance coverage and pricing can vary between the two treatments. Both medications may be covered by insurance, but patient access programs and manufacturer assistance can influence out-of-pocket costs. Patients should consult with their healthcare providers and insurance companies to determine the most cost-effective option.
Benefits of Brixadi for opioid use disorder treatment
One of the primary benefits of Brixadi is its ability to provide a consistent release of medication, maintaining stable levels in the body, and reducing cravings and withdrawal symptoms. This steady-state level is crucial for patients, as it minimizes the daily burden of medication management, enhancing adherence to treatment. Additionally, how Brixadi interacts with other medications can further influence treatment outcomes. The Brixadi REMS program is also an essential component of the treatment process.
The convenience of weekly or monthly dosing options further supports patient adherence, reducing the likelihood of missed doses and improving overall treatment outcomes. Additionally, the injection format offers greater privacy and less stigma, making it easier for patients to manage their treatment discreetly.
Brixadi offers several advantages that enhance its safety and effectiveness:
- Lower potential for misuse compared to oral formulations, enhancing its safety profile.
- Contains buprenorphine containing products as a partial agonist with a ceiling effect that reduces overdose risks.
- Clinical trials have demonstrated its effectiveness in reducing illicit opioid use.
These factors underscore Brixadi’s role in supporting long-term recovery.
Risks and side effects of Brixadi
Like any medication, Brixadi comes with potential risks and side effects. Most common adverse reactions include:
- Injection site reactions
- Headaches
- Insomnia
These side effects can last from a few days to weeks. Patients should be aware of these potential discomforts and discuss them with their healthcare providers.
More serious risks include adrenal insufficiency, central nervous system depression, and potentially fatal respiratory depression if misused. A boxed warning from the FDA highlights the risk of serious harm or death if Brixadi is injected intravenously, as it can form a gel that leads to serious harm from dangerous blood clots.
Patients should be cautious of the following when using Brixadi:
- Respiratory depression, especially when combined with other depressants like alcohol or benzodiazepines.
- Allergic reactions can range from mild symptoms like rashes to severe symptoms such as swelling and difficulty breathing.
- Individuals with asthma or liver issues should consult their healthcare providers before starting Brixadi to mitigate the risk of adverse effects.
How Brixadi is administered
Brixadi must be administered by a healthcare provider, ensuring that safety protocols are followed. The medication is administered via a subcutaneous injection, with the following guidelines:
- Never inject intravenously, intramuscularly, or intradermally.
- Injection sites include the buttock, thigh, abdomen, or upper arm. Injection site pruritus can occur, so it is important to rotate among these sites to minimize irritation and the risk of injection site erythema. Additionally, it is crucial to administer brixadi intravenously only under the supervision of a qualified healthcare provider, following proper intravenous administration protocols.
The injection process involves preparing the medication, ensuring the correct dosage, and administering it slowly into the subcutaneous tissue to ensure proper absorption. For patients transitioning from other buprenorphine products, a test dose of transmucosal buprenorphine is recommended before starting Brixadi.
After the injection, patients may need to be observed for a short period to monitor for any allergic reaction. Regular follow-up appointments are essential to ensure the effectiveness and safety of the treatment.
Cost of Brixadi and insurance coverage
The cost of Brixadi can vary depending on whether the patient has insurance. With insurance, out-of-pocket costs may be significantly reduced, but without insurance, the costs can be substantial. Patients should check with their insurance providers to understand their coverage options and potential out-of-pocket expenses.
Brixadi is covered by many insurance providers, and Braeburn Pharmaceuticals offers manufacturer-sponsored assistance programs to help reduce costs for eligible patients. Comparing the costs of Brixadi with other OUD treatments like Suboxone and Sublocade can help patients and providers make informed decisions.
Cost considerations are crucial in choosing a treatment plan, and patients should discuss financial aspects with their healthcare providers to ensure they receive the most effective and affordable care.
Patient experiences and outcomes with Brixadi
Real-world data on Brixadi shows high levels of patient satisfaction and adherence. Patients using subcutaneous buprenorphine ER, like Brixadi, reported higher satisfaction compared to those on sublingual buprenorphine, with an equivalent buprenorphine exposure. Clinical trials have shown that 68% of patients switching to Brixadi felt it was significantly better than their previous treatment.
Patients have expressed satisfaction with Brixadi due to:
- Its ability to manage cravings and withdrawal symptoms
- Reported improvements in their quality of life, including better relationships and self-care
- The convenience of less frequent dosing, which contributed to a lower medication burden and greater adherence, prevented precipitated withdrawal.
Retention rates for patients on Brixadi were high, with 74% completing a 48-week treatment study. While some patients experienced challenges during the transition, the overall patient experience with Brixadi has been positive, supporting its role in effective OUD treatment.
Alternatives to Brixadi for opioid use disorder
Several suboxone alternatives to Brixadi are available for treating opioid use disorder, each with its own mechanisms, dosing, benefits, and risks. Suboxone, a combination of buprenorphine and naloxone, is a commonly used sublingual tablet that helps reduce cravings and withdrawal symptoms. Sublocade, another extended-release buprenorphine injection, is administered monthly and offers similar benefits to Brixadi but without the weekly dosing option.
Methadone is a full opioid agonist that is administered daily under supervision, making it a suitable option for patients who need close monitoring. Naltrexone, an opioid antagonist, blocks the effects of opioids and is available in both oral and injectable formulations. Each of these treatments has its unique advantages and potential risks, and healthcare providers play a crucial role in determining the most appropriate option based on individual patient needs, including the use of opioid agonists.
Choosing the right treatment involves considering factors such as the severity of the disorder, patient preferences, and potential side effects. A comprehensive approach, including a complete treatment plan, behavioral therapy, psychosocial support, and mental health services administration, is essential for effective treatment.
Bottom Line: Is Brixadi right for you?
Deciding whether Brixadi is the right treatment for you involves a thorough discussion with your healthcare provider. Important considerations include:
- Brixadi may not be suitable for individuals with severe liver issues.
- It is not suitable for those who have a known allergy to buprenorphine.
- Avoid combining Brixadi with alcohol or sedative medications to reduce the risk of respiratory problems.
Patients should consider their treatment goals, lifestyle, and preferences when discussing Brixadi with their healthcare providers. Regular follow-up appointments are crucial to ensure the effectiveness and safety of the treatment.
By engaging in shared decision-making, patients and providers can determine if Brixadi is the best fit for their recovery journey.
Frequently asked questions about Brixadi
Can you switch from Suboxone to Brixadi?
You can switch from Suboxone to Brixadi, but it is crucial to consult with a healthcare provider for a smooth transition and to manage any potential withdrawal symptoms.
Is Brixadi available at most treatment centers?
Brixadi is increasingly available at treatment centers, but it is advisable to confirm its availability with local facilities.
How long does it take for Brixadi to start working?
Brixadi usually starts to take effect within a few hours after administration. Therefore, you can expect to feel its effects relatively quickly.
What should I do if I miss a Brixadi dose?
If you miss a dose of Brixadi, it is essential to contact your healthcare provider promptly to determine the appropriate course of action.
Are there any serious risks associated with Brixadi?
Yes, Brixadi poses serious risks such as respiratory depression and adrenal insufficiency, with potentially fatal outcomes if injected intravenously. It is crucial to adhere to your healthcare provider’s guidance and report any adverse effects promptly.