Suboxone came out in 2002 after FDA approval, offering a new way to treat opioid addiction. This article will explore its development, approval, and impact, including the question of when did Suboxone come out.
Key Takeaways
- Suboxone was approved by the FDA in 2002, marking a significant advancement in treatment options for opioid dependence, facilitated by the Drug Addiction Treatment Act of 2000.
- Developed by Reckitt Benckiser, Suboxone combines buprenorphine and naloxone, targeting opioid addiction by minimizing misuse while alleviating withdrawal symptoms.
- Suboxone’s introduction changed addiction treatment protocols by allowing prescriptions in office-based settings, thereby expanding access and reducing stigma for patients seeking help.
When was Suboxone approved and released for public use?
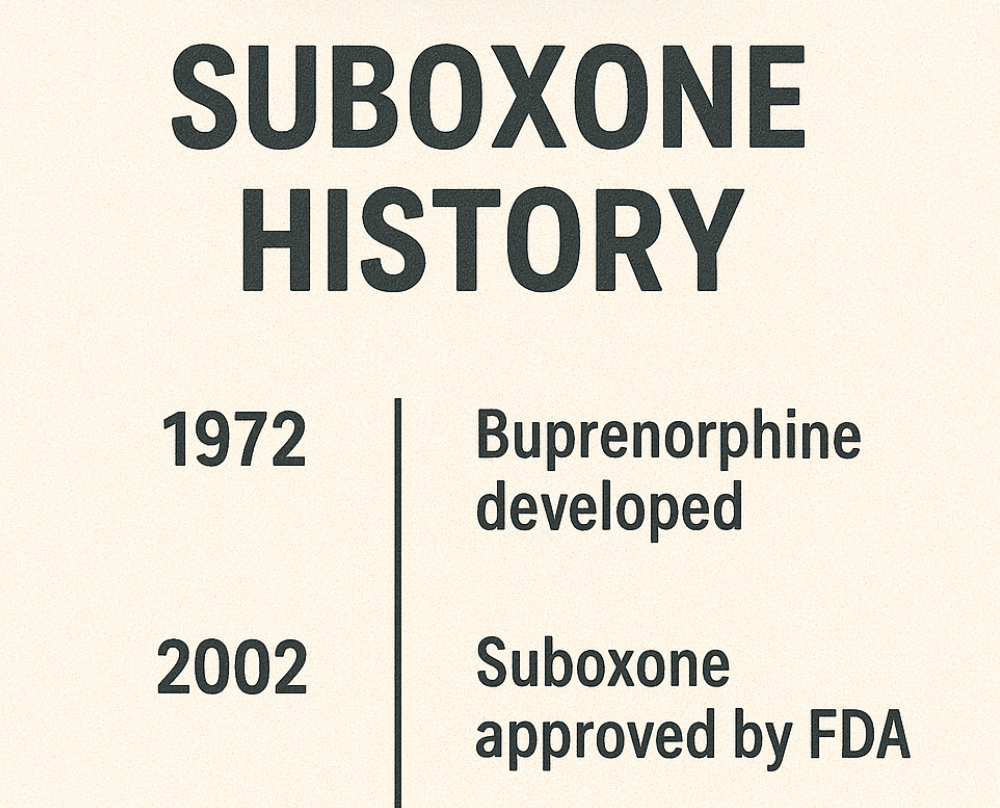
The journey of Suboxone began with its approval by the Food and Drug Administration (FDA) in 2002. This approval was a significant milestone, as it marked the introduction of a new and effective treatment option for opioid dependence. Suboxone, a medication that combines buprenorphine and naloxone, was designed to provide a safer alternative to traditional opioid treatments.
The Drug Addiction Treatment Act of 2000 (DATA 2000) facilitated Suboxone’s approval, permitting buprenorphine-containing medications to be prescribed outside specialized opioid treatment programs. This was a groundbreaking change, as it enabled physicians to prescribe Suboxone in office-based settings, making treatment more accessible to patients. The FDA’s endorsement underscored the rigorous evaluation of Suboxone’s efficacy, safety, and classification. Learn more about Suboxone is a controlled substance, and what that means for patients.
Reckitt Benckiser Pharmaceuticals was instrumental in Suboxone’s development and approval, ensuring the medication met all regulatory requirements through close collaboration with the FDA. This approval marked a significant step forward in combating opioid addiction, benefiting both the pharmaceutical company and public health. The participation of the Substance Abuse and Mental Health Services Administration (SAMHSA) and the Drug Enforcement Administration (DEA) highlighted the medication’s importance in addressing the opioid crisis.
Suboxone’s regulatory approval significantly expanded treatment access, allowing prescriptions outside of opioid treatment programs and offering a more convenient, less stigmatized option for patients. This regulatory milestone ushered in a new era in addiction treatment, providing hope to those battling opioid use disorder.
Who developed Suboxone and why?
Suboxone’s development represents innovation and dedication to tackling a growing public health crisis. Reckitt Benckiser, a global leader in consumer health and hygiene, is the pharmaceutical company behind Suboxone. John Lewis initiated the development of Suboxone in 1966 as part of efforts to create improved analgesics. Over the years, the vision for Suboxone evolved significantly.
Suboxone was created to offer a safer option for pain relief and to tackle the growing issue of opioid addiction. Combining buprenorphine, a partial opioid agonist, with naloxone, an opioid antagonist, and buprenorphine naloxone, Suboxone was specifically designed to reduce misuse and overdose potential in opioid addiction treatment. This unique combination aimed to provide effective treatment while minimizing the risks associated with other opioid medications.
The escalating opioid crisis in the 1990s and early 2000s significantly motivated Suboxone’s development. Key factors include:
- The surge in heroin and prescription opioid abuse created an urgent need for effective and safe treatment options.
- Research in the 1970s led to the creation of buprenorphine as a partial opioid agonist, enhancing safety in pain management.
- This research laid the foundation for developing Suboxone as a treatment for opioid use disorder, highlighting suboxone’s history.
The National Institute on Drug Abuse (NIDA) and the Substance Abuse and Mental Health Services Administration (SAMHSA) played pivotal roles in promoting alternatives to the then-standard methadone diversion treatment. While effective, methadone had significant drawbacks, such as potential misuse, abuse potential, and the need for daily clinic visits. NIDA and SAMHSA’s support for buprenorphine and naloxone combinations like Suboxone was crucial in advancing this new addiction therapeutic option.
In summary, Suboxone’s development responded to the heroin crisis and the broader opioid epidemic. It represented a significant advancement in addiction medicine, offering safer and more accessible treatment for opioid dependence. Reckitt Benckiser’s commitment, along with support from key organizations, made Suboxone a vital tool in the fight against opioid addiction.
What made Suboxone a turning point in addiction treatment?
Suboxone signifies a pivotal moment in addiction treatment history, owing to its unique pharmacological profile and the accompanying policy changes. The medication combines buprenorphine combined, a partial opioid agonist, with naloxone, an opioid antagonist. This combination helps alleviate withdrawal symptoms and cravings while reducing misuse potential, especially in the context of narcotic drugs. Buprenorphine provides the benefits of opioid treatment without the same level of risk as full opioid agonists.
A significant change brought by Suboxone was its ability to be prescribed in office-based settings, enabled by the Drug Addiction Treatment Act of 2000 (DATA 2000). This legislation allowed qualified physicians to prescribe buprenorphine-containing medications, like Suboxone, outside specialized opioid treatment programs. This monumental shift expanded access to addiction treatment, making it more convenient and reducing the stigma of seeking help.
Expanded access meant more patients could receive care from their primary care physicians instead of visiting specialized clinics. This was especially beneficial for individuals in rural or underserved areas with limited access to specialized addiction treatment facilities. Receiving treatment in an outpatient setting also allowed patients to maintain their daily routines and responsibilities, crucial for long-term recovery in various treatment settings. However, it is important to note that some patients may be at a higher risk for complications during their recovery process.
Early adoption trends indicated receptiveness to Suboxone from both physicians and patients. The medication quickly gained popularity as an effective treatment for opioid dependence, with many patients experiencing significant quality of life improvements. Suboxone’s convenience and efficacy made it a preferred choice for many, solidifying its role as a turning point in addiction treatment.
In summary, Suboxone’s introduction marked a significant shift in opioid addiction treatment. Its unique formulation and policy changes permitting office-based prescribing expanded treatment access and reduced addiction stigma. This turning point in addiction medicine has given countless individuals the chance for recovery and a better quality of life.

Key milestones in Suboxone’s history after release
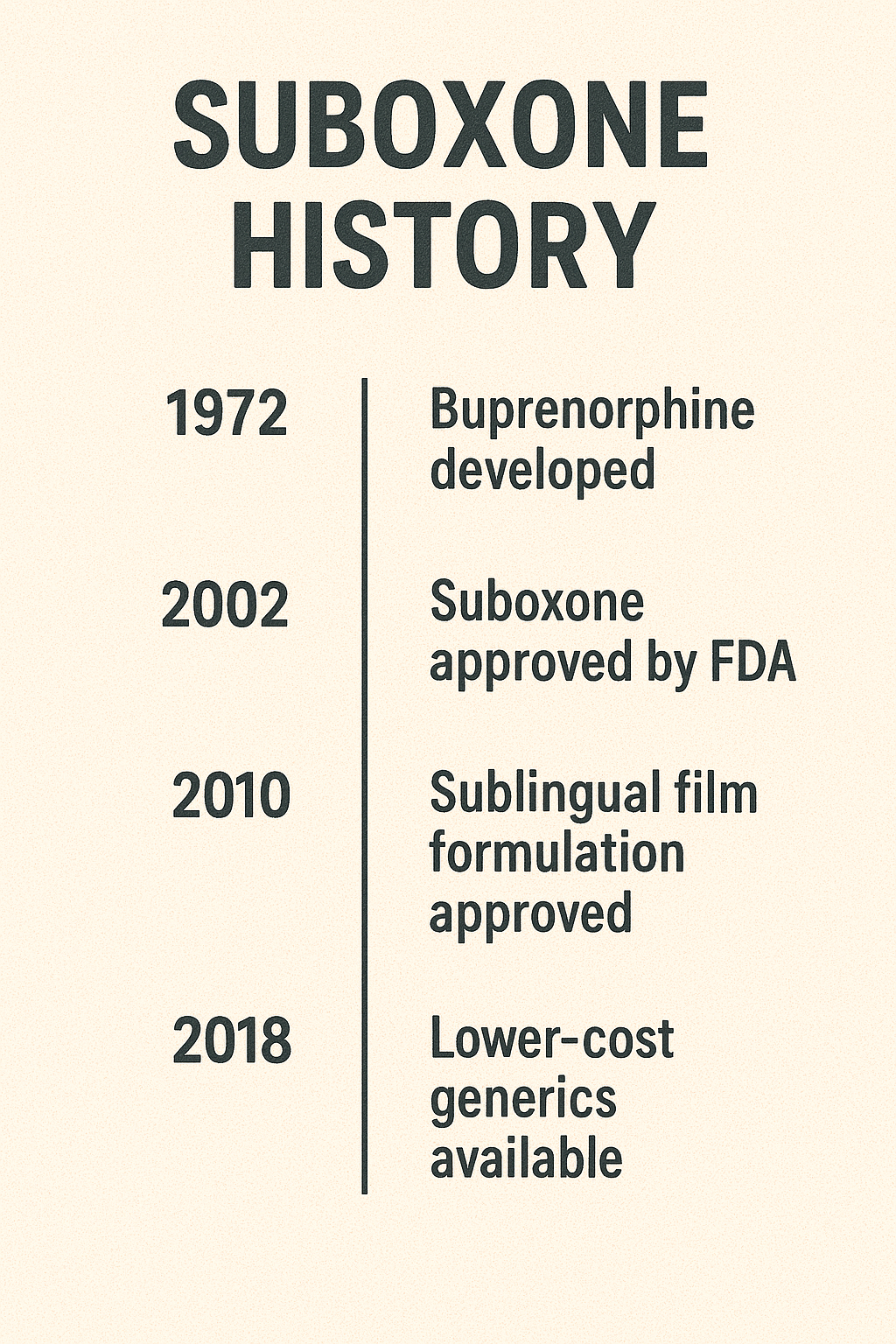
Suboxone’s journey continued post-approval, achieving significant milestones that further cemented its place in addiction treatment. An early milestone was the rise in physicians receiving waivers to prescribe buprenorphine between 2003 and 2005. This increase in prescribing authority was crucial for expanding access to Suboxone and ensuring more patients could benefit.
In 2010, introducing generic versions and alternative formulations of Suboxone marked another important milestone. These alternatives made the medication more accessible and affordable, ensuring cost wouldn’t be a barrier to treatment. The availability of these formulations provided options for patients with varying needs and preferences.
The 2012 release of Suboxone Film aimed at improving patient adherence was a significant development. The film formulation was designed to dissolve quickly and be less prone to misuse than the tablet form. This innovation addressed medication adherence challenges and further enhanced Suboxone treatment effectiveness.
From 2016 onward, the Comprehensive Addiction and Recovery Act (CARA) expanded Suboxone’s prescribing authority to include nurse practitioners and physician assistants. This expansion was crucial for addressing the shortage of qualified prescribers and increasing patient access to treatment. Involving these healthcare professionals broadened Suboxone’s reach, making it more accessible in various settings.
Each of these milestones significantly shaped Suboxone’s history and its impact on addiction treatment. Suboxone’s continued evolution and adaptation have ensured it remains vital in combating opioid addiction, offering hope and recovery to countless individuals.
How has Suboxone evolved since it came out?
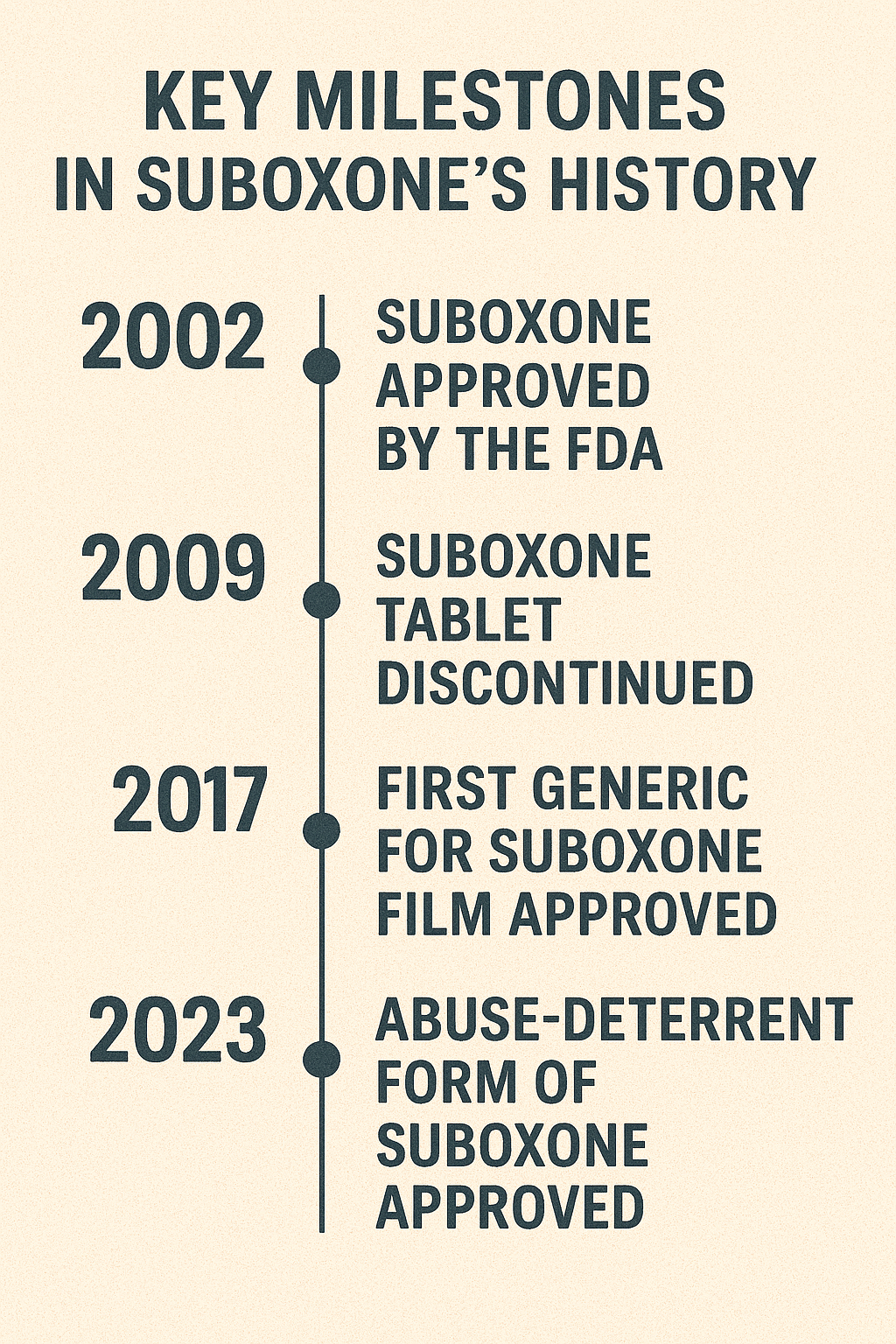
Since its introduction, Suboxone has evolved significantly in terms of formulations, brand ownership, and regulatory adaptations. Developing new formulations, such as tablets, films, and injections, has been a critical aspect of this evolution. These formulations cater to various patient needs and preferences, enhancing overall treatment effectiveness.
Changes in brand ownership and manufacturing have also been part of Suboxone’s evolution. These changes have influenced the medication’s availability and distribution, ensuring it remains accessible to patients. Various companies’ commitment to maintaining Suboxone’s quality and availability has been crucial to its success.
However, Suboxone’s journey hasn’t been without controversies. Instances of litigation and debates over marketing practices and diversion risks have occurred. These controversies have prompted ongoing discussions about managing and regulating Suboxone to minimize risks and adverse effects while maximizing benefits.
Patient education and prescribing regulations have evolved to ensure Suboxone’s safe and effective use. Healthcare providers have adapted practices to better educate patients on the proper use and potential misuse risks of Suboxone. These adaptations have been essential in promoting responsible Suboxone use and improving patient outcomes.
Overall, Suboxone’s evolution reflects ongoing efforts to enhance effectiveness and accessibility while addressing associated challenges and risks. This continuous improvement ensures that Suboxone remains a cornerstone in addiction treatment, providing hope and recovery for those struggling with opioid dependence.
How Suboxone compares to earlier and newer treatments
Suboxone’s introduction marked a significant advancement in the treatment of opioid dependence, offering several advantages compared to Suboxone vs methadone as earlier treatments. Methadone, the prior standard of care, was effective but had significant drawbacks, including a high potential for misuse and the requirement for daily clinic visits. Suboxone, with its partial opioid agonist properties, offered a safer and more accessible alternative.
Compared to newer treatments, Sublocade vs Suboxone presents its own unique benefits and challenges in the context of opioid treatment. Sublocade, an extended-release injection of buprenorphine, offers the advantage of less frequent dosing, which can improve adherence for some patients. However, Suboxone’s sublingual buprenorphine and naloxone combination remains a preferred option for many due to its efficacy and the flexibility it offers in dosing.
Suboxone’s partial opioid agonist properties, which include both agonist and antagonist effects, provide a balanced approach to treating opioid dependence. This balance helps manage withdrawal symptoms and cravings while reducing the risk of precipitate withdrawal compared to full agonist opioid agonists and receptor occupancy. The accessibility of Suboxone, thanks to DATA 2000, further sets it apart from treatments that require more stringent regulatory oversight, including those involving two opioid agonists and opioid receptors.
In terms of administration methods, Suboxone offers versatility with its various formulations, including tablets, films, and injections. This versatility allows healthcare providers to tailor treatment to individual patient needs, enhancing the overall effectiveness of the medication.
Overall, Suboxone stands out as a versatile and effective treatment to treat opioid dependence. Its unique properties, combined with the flexibility in administration and expanded access, make it a valuable tool in both the historical and modern context of addiction treatment.
Why understanding Suboxone’s release date matters for patients today
Understanding the release date of Suboxone is more than just historical trivia; it holds significant implications for patients and healthcare providers today. The approval of Suboxone in 2002 marked a pivotal shift in how opioid dependence could be treated, moving away from the confines of specialized clinics to more accessible, office-based settings. This change made treatment more convenient and less stigmatizing, encouraging more individuals to seek help.
Knowledge of Suboxone’s timeline can also inform patient trust and expectations. Being aware that Suboxone has been in use for over two decades, backed by extensive research and clinical experience, can provide reassurance to patients considering this treatment option. It highlights the medication’s long-standing efficacy and safety profile, fostering confidence in its use.
Linking this historical understanding to present-day treatment trends, we see a growing acceptance of medication-assisted treatment (MAT) as a standard approach to managing opioid dependence. The historical context of Suboxone’s release helps explain why MAT, including Suboxone and maintenance treatment, is now widely endorsed and integrated into various treatment protocols. This broader acceptance is crucial for reducing the stigma associated with addiction and promoting comprehensive, evidence-based care.
In summary, understanding when Suboxone came out and the context of its release helps patients appreciate the evolution and reliability of their treatment options. It underscores the progress made in addiction medicine and reinforces the importance of accessible, effective treatments in the ongoing battle against the opioid crisis.
Bottom Line: The legacy of Suboxone since its release
The legacy of Suboxone since its release in 2002 is profound and far-reaching. As a cornerstone in medication-assisted treatment for opioid dependence, Suboxone has significantly reduced opioid overdose deaths and improved the quality of life for countless individuals. Its unique combination of buprenorphine and naloxone was specifically designed to mitigate the likelihood of misuse, setting it apart from earlier treatments.
Suboxone’s impact extends beyond individual patient outcomes; it has influenced addiction treatment policies and practices, promoting harm reduction strategies and expanding access to care. The ability to prescribe Suboxone in office-based settings, thanks to DATA 2000, has made treatment more accessible and less stigmatizing, encouraging more people to seek help.
Since its release, Suboxone has been recognized for its role in improving the quality of life for those recovering from opioid addiction. The medication has provided a reliable and effective treatment option, helping individuals regain stability and rebuild their lives. The ongoing research into Suboxone’s efficacy in various populations and conditions continues to enhance our understanding and application of this vital medication.
As we look to the future, the legacy of Suboxone will undoubtedly continue to evolve. Ongoing advancements in addiction medicine and continued support for medication-assisted treatment will ensure that Suboxone remains a critical tool in the fight against opioid addiction. For those seeking recovery, Suboxone offers hope and a pathway to a healthier, more stable life.
Frequently asked questions about when Suboxone came out
When was Suboxone first approved by the FDA?
Suboxone was first approved by the FDA in 2002.
Why was Suboxone released in 2002?
Suboxone was released in 2002 to combat the escalating opioid crisis by offering a safer and more accessible treatment option for individuals struggling with opioid dependence. Its introduction aimed to improve treatment outcomes and reduce the harms associated with opioid use.
How long has Suboxone been in use for opioid addiction?
Suboxone has been utilized for opioid addiction since its FDA approval in 2002, making it a well-established treatment option for over two decades.
Who invented Suboxone?
Suboxone was developed by Reckitt Benckiser Pharmaceuticals, initiated by John Lewis in 1966. This medication is used to treat opioid addiction effectively.
Is Suboxone still considered a first-line treatment today?
Suboxone is still considered a first-line treatment for opioid dependence today, owing to its proven effectiveness and safety profile.